Sinopharm Vaccine Wikipedia / Uae Announces Sinopharm Vaccine Has 86 Efficacy Against Covid 19 Nikkei Asia
The candidate is said to be the world's first inactivated vaccine to enter a phase iii trial. It completed phase iii trials in argentina, bahrain, egypt, morocco, pakistan, peru, and the united arab emirates (uae) with over 60,000 participants. The company sinopharm belongs to the government of china. On december 29, 2020, sinopharm reported 79% efficacy in an interim evaluation. Sinopharm group researches and develops, manufactures, distributes, and markets medicine and other healthcare products. It completed phase iii trials in argentina, bahrain, egypt. This trial is being conducted by sinopharm's china national biotec group (cnbg) in alliance with the. As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective.
This vaccine does not have a name. Sinopharm group researches and develops, manufactures, distributes, and markets medicine and other healthcare products. Sinopharm's vaccines are also being used in the united arab emirates and bahrain (beijing vaccine). In july, the ministry of health also allowed a company in ho chi minh city to import 5 million doses of sinopharm vaccine. What you need to know.
As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective.
This vaccine started phase iii trials in the middle of july 2020. This chemical binds to the virus's genetic material and stops it from. Sinopharm's vaccines are also being used in the united arab emirates and bahrain (beijing vaccine). By may, sinopharm had supplied 200 million doses. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus that causes coronavirus disease 2019 ().prior to the covid‑19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (sars) and middle. What you need to know. On 7 may 2021, the world health organization approved the vaccine for use in covax. Sinopharm vaccine will be administered to chinese nationals in vietnam, those wishing to go to, study or work in china and those. Currently, sinovac is still pursuing its application for clinical trials in the philippines. On december 29, 2020, sinopharm reported 79% efficacy in an interim evaluation. Information from its description page there is. As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective. What you need to know.
This is a file from the wikimedia commons. As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective. This vaccine started phase iii trials in the middle of july 2020. On 7 may 2021, the world health organization approved the vaccine for use in covax. Sinopharm vaccine will be administered to chinese nationals in vietnam, those wishing to go to, study or work in china and those. This article provides a summary of the interim recommendations;
On 7 may 2021, the world health organization approved the vaccine for use in covax.
As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective. Here is what you need to know. On 7 may 2021, the world health organization approved the vaccine for use in covax. By may, sinopharm had supplied 200 million doses. 289 × 240 pixels | 577 × 480 pixels | 722 × 600 pixels | 924 × 768 pixels | 1,239 × 1,030 pixels. It completed phase iii trials in argentina, bahrain, egypt, morocco, pakistan, peru, and the united arab emirates (uae) with over 60,000 participants. On 7 may 2021, the world health organization approved the vaccine for use in covax. This trial is being conducted by sinopharm's china national biotec group (cnbg) in alliance with the. How the sinopharm vaccine works. What you need to know. This vaccine does not have a name. Sinopharm vaccine will be administered to chinese nationals in vietnam, those wishing to go to, study or work in china and those. On december 29, 2020, sinopharm reported 79% efficacy in an interim evaluation. By may, sinopharm had supplied 200 million doses.
It completed phase iii trials in argentina, bahrain, egypt. This is a file from the wikimedia commons. By may, sinopharm had supplied 200 million doses. Sinopharm's vaccines are also being used in the united arab emirates and bahrain (beijing vaccine). The candidate is said to be the world's first inactivated vaccine to enter a phase iii trial. What you need to know. By may, sinopharm had supplied 200 million doses.
Here is what you need to know.
On december 29, 2020, sinopharm reported 79% efficacy in an interim evaluation. The candidate is said to be the world's first inactivated vaccine to enter a phase iii trial. How the sinopharm vaccine works. The interim recommendations and the background document are also available online. Sinopharm vaccine will be administered to chinese nationals in vietnam, those wishing to go to, study or work in china and those. What you need to know. On 7 may 2021, the world health organization approved the vaccine for use in covax. By may, sinopharm had supplied 200 million doses. This chemical binds to the virus's genetic material and stops it from. This vaccine started phase iii trials in the middle of july 2020. Here is what you need to know. As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective.
It completed phase iii trials in argentina, bahrain, egypt sinopharm vaccine. By may, sinopharm had supplied 200 million doses.
Here is what you need to know.

This vaccine started phase iii trials in the middle of july 2020.

This article provides a summary of the interim recommendations;

This article provides a summary of the interim recommendations;

Sinopharm vaccine will be administered to chinese nationals in vietnam, those wishing to go to, study or work in china and those.

As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective.

289 × 240 pixels | 577 × 480 pixels | 722 × 600 pixels | 924 × 768 pixels | 1,239 × 1,030 pixels.
What you need to know.

3 sinopharm vaccine waste management • used vials and syringes must be collected safely by the vaccinator • syringes must be disposed through environment friendly incinerators where available • the vials and syringes must be disposed together in burial/burning pits • pit must be closed with 50cm (half ft.) soil layer before the pit is completely

This vaccine started phase iii trials in the middle of july 2020.

It completed phase iii trials in argentina, bahrain, egypt, morocco, pakistan, peru, and the united arab emirates (uae) with over 60,000 participants.

This vaccine does not have a name.

What you need to know.

This vaccine does not have a name.

In july, the ministry of health also allowed a company in ho chi minh city to import 5 million doses of sinopharm vaccine.
Sinopharm vaccine will be administered to chinese nationals in vietnam, those wishing to go to, study or work in china and those.

The candidate is said to be the world's first inactivated vaccine to enter a phase iii trial.

On 7 may 2021, the world health organization approved the vaccine for use in covax.

Information from its description page there is.
This chemical binds to the virus's genetic material and stops it from.
The interim recommendations and the background document are also available online.

By may, sinopharm had supplied 200 million doses.

What you need to know.

This vaccine started phase iii trials in the middle of july 2020.
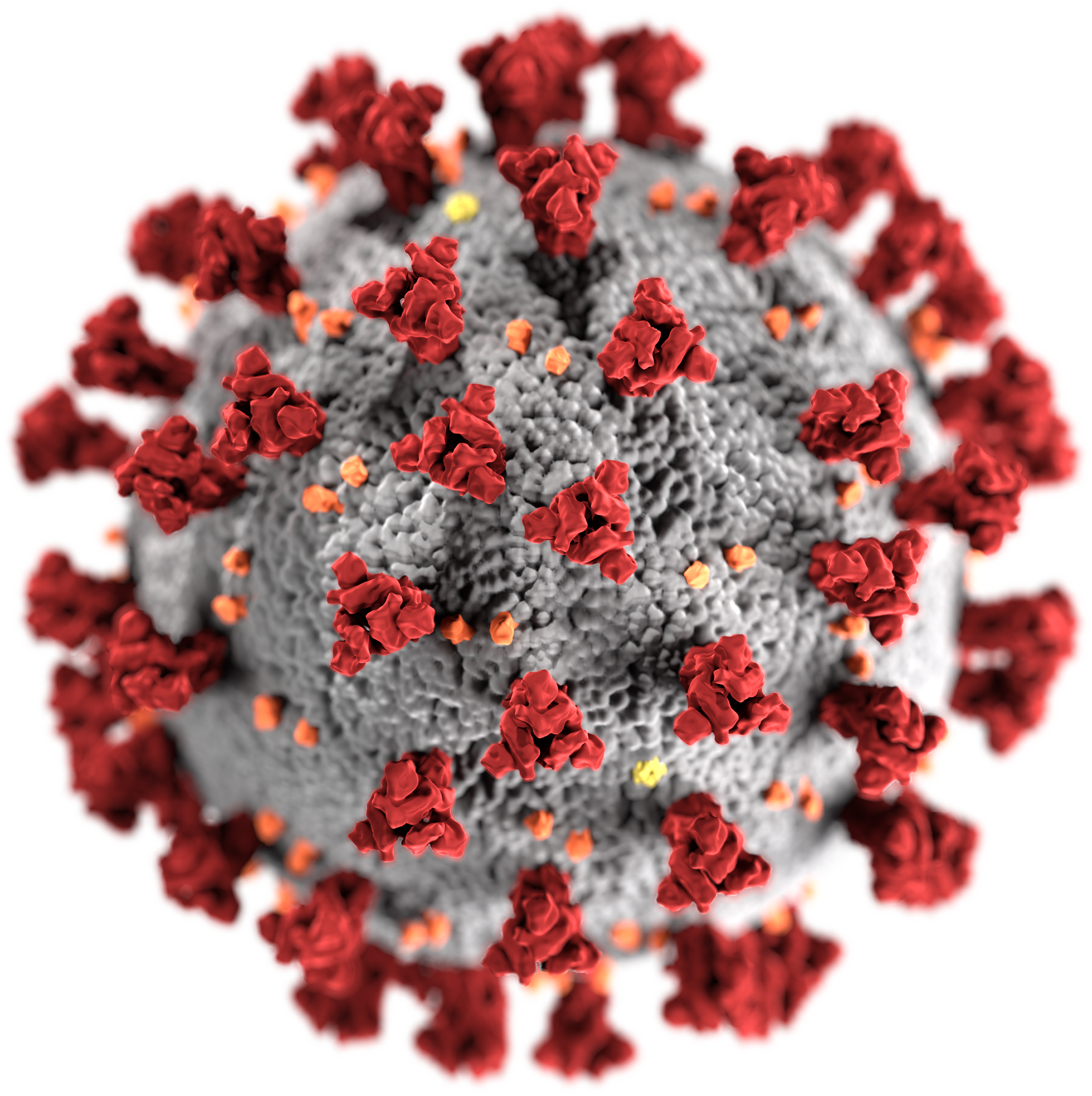
A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus that causes coronavirus disease 2019 ().prior to the covid‑19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (sars) and middle.
Sinopharm's vaccines are also being used in the united arab emirates and bahrain (beijing vaccine).

What you need to know.
The interim recommendations and the background document are also available online.

3 sinopharm vaccine waste management • used vials and syringes must be collected safely by the vaccinator • syringes must be disposed through environment friendly incinerators where available • the vials and syringes must be disposed together in burial/burning pits • pit must be closed with 50cm (half ft.) soil layer before the pit is completely

The interim recommendations and the background document are also available online.

As part of the company's phase 3 trials, filipinos will be able to see if the vaccine is safe and effective.

It completed phase iii trials in argentina, bahrain, egypt, morocco, pakistan, peru, and the united arab emirates (uae) with over 60,000 participants.
This article provides a summary of the interim recommendations;

This is a file from the wikimedia commons.
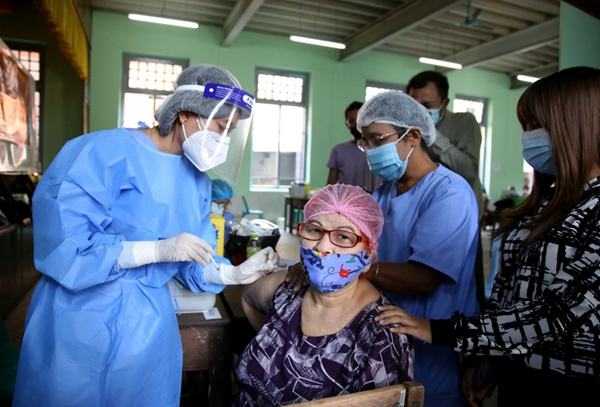
This vaccine does not have a name.

289 × 240 pixels | 577 × 480 pixels | 722 × 600 pixels | 924 × 768 pixels | 1,239 × 1,030 pixels.



